Inhalt
Prof. Sigrun Korsching
Institute for Genetics
+49 221 470 4843
+49 221 470 5172
Project proposal: The role of a de-orphanized olfactory receptor in neurogenesis and axonal pathfinding
The sense of smell is essential for food detection, prey and predator recognition, reproduction and other intraspecies communication. Several olfactory receptor gene families, both small and large ones, contribute to the detection of odors. We have recently characterized one of these olfactory receptor gene families, the TAAR genes, in lower vertebrates 1 and found a series of physiologically relevant ligands for a receptor from this family that elicit aversive behavior in close correspondence to their effectiveness as ligands. These ligands also activate a sparse subset of olfactory receptor neurons (see figure). Thus we have identified a defined neural circuit that spans from peripheral sensory detection via a single receptor to generation of specific behavior. This is a novum for any vertebrate species and constitutes an excellent basis to study neuronal development and plasticity in a defined neural circuit. Intriguingly, olfactory receptor molecules are not only involved in odor perception, but also appear to serve double duty in axonal pathfinding of olfactory sensory neurons, as has been examined in rodents 2. We suggest to study this phenomenon in the functionally identified neural circuit described above, using zebrafish, Danio rerio, as experimental system. Zebrafish is an established model system for studying olfaction and has many advantages especially for studying early development.

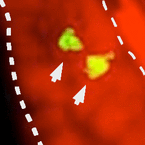
Figure: Left panel, in situ hybridization for the TAAR receptor shows sparse expression pattern in the olfactory sensory surface (aka nose) typical for olfactory receptor genes. Two receptor neurons expressing the TAAR receptor are seen (red arrows). Right panel, TAAR ligand-induced neuronal activity shows very similar sparse pattern of activated neurons, nuclear dye (red) visualizes the tissue. Dashed lines delimit the olfactory epithelium, lumen is to the left.
In this project we will make use of modern genetic techniques to fully characterize the neuronal circuit activated by the TAAR receptor. Chimeric constructs expressing both the taar gene and a fluorescent reporter gene (2A technique) will serve to identify the target glomerulus. Co-expression of a transsynaptic marker, e.g. WGA lectin, will serve to identify the neurons innervated by the receptor neurons. Further levels of the pathway can be identified by expressing a reporter gene under activity-regulated promoter control (c-fos). In parallel we will be applying knockout and/or knockdown techniques to confirm the involvement of the TAAR receptor in the innate behavior generated by its ligand.
Using these results we will ask essential questions about the development and plasticity of a defined neuronal circuit: Is the activity of the olfactory receptor required for proper targeting? When is the circuit functional? Does the function of the pathway change during development (attraction vs aversion)? Does exposure to the ligand modify the identified circuit (adaptation or sensitization, changes in neurogenesis or survival)?
