Inhalt
Prof. Dr. Guenter Schwarz
Institute of Biochemistry
Mechanistic control and functional importance of alternative gephyrin splicing
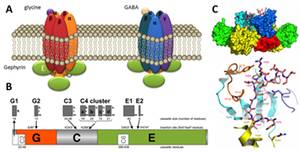
Anchoring and clustering of neuroreceptors is vital for proper signal transmission in the central nervous system. The protein gephyrin plays a critical role in organizing postsynaptic structures at glycinergic and GABAergic synapses (Fig. 1). Gephyrin is a multifunctional protein that binds to the glycine receptor (GlyR) and was found to be an instructive molecule for postsynaptic clustering of inhibitory glycine and GABAA receptors (Kirsch and Betz, 1998). Gephyrin is a 93 kDa multi-domain protein composed of an N-terminal 20 kDa G-domain, a central domain and a C-terminal 48 kDa E-domain (Fritschy et al., 2008) (Fig. 1B). The modular nature of gephyrin is derived from its evolutionary origin in basic metabolism, catalyzing the terminal steps in the biosynthesis of the molybdenum cofactor (Schwarz et al., 2009).
Gephyrin binds to the large cytoplasmic loop of the GlyR b-subunit (GlyRb-loop, Fig. 1C) with high affinity via its E-domain (Schrader et al., 2004) and the structural basis of a key-and-lock mechanism of interaction between the receptor and GephE has been uncovered (Kim et al., 2006). Beside the interaction with inhibitory receptors, gephyrin binds to a number of signaling, cytoskeletal and motorprotein-associated proteins that are thought to control gephyrin-mediated transport and clustering of GlyR and GABAA receptors (Fritschy et al., 2008).
Figure 1: Inhibitory receptors and gephyrin. (A) Glycine and GABA type A receptors form pentameric chloride channels. Their synptic orgsanization is dependent on gephyrin. (B) Domain structure and alternatively spliced exons in gephyrin. (C) Structure of gephyrin E domain in complex with the interacting peptide of the GlyR b-loop (Kim et al., 2006).
Functional diversity of gephyrin is believed to reside in its complex alternative splicing resulting in at least 10 different variants expressed in brain and peripheral tissue (Fritschy et al., 2008). We investigated gephyrin splice variants from different model systems (Ogino et al., 2010) and found that all modifications affecting the central domain do not impact gephyrin’s metabolic function. Recently, we investigated in collaboration with Prof. Jochen Meier (MDC Berlin) gephyrin expression in the hippocampus of patients with intractable temporal lobe epilepsy (TLE). Immunohistochemical and Western blot analyses revealed irregular gephyrin expression in the cornu ammonis of those patients, and four abnormally spliced gephyrins lacking several exons in their G-domains were isolated (Forstera et al., 2010). Identified TLE gephyrins have oligomerisation deficits, and they curtail hippocampal postsynaptic gephyrin and GABA type A receptor ?2 while interacting with regularly spliced gephyrins. We found that cellular stress (alkalosis and hyperthermia) causes association of the neuronal splice factor NeuN with ubiquitin and inhibits gephyrin RNA splicing providing a novel mechanism of stress-related splicing errors in gephyrin.
The aim of the project is to understand the mechanism that controls tissue specific gephyrin splicing and how this process is affected under pathological conditions. In particular we wish to understand the underlying mechanism of dominat negative oligomerizations of miss-spliced gephyrin on a functional and structural level.
The project includes:
- expression studies of gephyrin splice variants in brain slices and cultured neurons
- functional analysis of gephyrin splice variants in respect to their oligomerization and binding to known interaction partners
- analysis of mouse models expressing TLE-gephyrins (in collaboration with Jochen Meier at the MDC Berlin)
- structural studies (X-ray crystallography) on gephyrin variants in order to identify interaction surfaces